Chemical Storage

Consult SDS, labels, CHP, supervisors, or EHS if you are unsure of proper storage of chemicals.
(See Chemical Compatibility Chart below).
- Flammable Liquids – Flammable liquids are required to be stored in flammable liquid storage cabinets approved by the National Fire Protection Association (NFPA) or flammable liquid storage rooms meeting OSHA requirements in 29 CFR 1910.106. OSHA's requirements include ventilation, dikes, explosion proof lighting, intrinsically safe wiring, grounding and bonding. Oxidizers, acids and other incompatible chemicals are prohibited from being stored in these areas. Do not permit sources of ignition in or near storage areas.
- Corrosives – Corrosives can be acidic or basic. Acids and bases should never be stored together. Corrosives should not be stored with flammable or combustible materials. Spill trays should be used to contain leaks.
- Oxidizers – Store in an isolated area away from flammable or combustible materials. These agents may react at room temperature producing fire or explosions. Do not mix strong oxidizers with combustible materials. Some are even explosive on contact with organic materials. Examples include perchloric acid, chromic acid, and hydrogen peroxide.
- Toxic and Poisonous Materials – Store in isolated areas, Do not store with acids or flammable materials.
- Cryogenic Liquefied Gases – Store in cool, well ventilated areas. Cryogenic gases boil off at room temperatures and must be vented to prevent dangerous excessive pressure build up. This vented gas can displace oxygen in enclosed or unventilated areas. The liquid form will instantly cause cold-contact burns to living tissue upon contact.
- Water Reactive Compounds – Store in isolated location away from any water sources.
- Pyrophoric Compounds – Store in isolated location under nitrogen.
- Peroxide Forming Compounds – Do not store with organics or solvents. Store in airtight containers in a dark, cool but not freezing, and dry area. Do not permit sources of heat, friction, grinding, or impact near storage areas. Date upon receiving and opening all incoming peroxide forming chemicals and dispose of them immediately upon reaching their expiration date. Some example of peroxide forming compounds are: diethyl ether, vinylidene chloride, sodium amide, styrene, tetrahydrofuran, and dioxane.
- Special Compounds – Follow specific storage instructions from chemical manufacturers. Check for moisture in the bottle of explosive chemicals that must be stored wet or in solution. Date all incoming shock sensitive explosive chemicals and dispose of them immediately upon reaching their expiration date. Both picric acid and benzoyl peroxide must be kept wet. If the solution dries, the crystals form very sensitive explosive compounds. Any shock or friction could set these off. Some chemicals like diethyl pyrocarbonate must be refrigerated to remain stable. Once unstable, removing the cap could cause an explosion. Do not mix combustibles with perchlorates. Many perchlorates become explosive when mixed with combustibles. Examples include: silver perchlorate, ammonium perchlorate, sodium perchlorate, and potassium perchlorate. Organic perchlorates like methyl perchlorate are self contained explosives.
- Compressed Gas Cylinders – Compressed gas cylinders must be secured in an upright position away from excessive heat, highly combustible materials, and areas where they might be damaged or knocked over. A chain, bracket or other restraining device shall be used to secure the cylinder at all times to prevent them from falling. The cylinder status as to "full" or "empty" must be indicated on the cylinder and the valve cap must be in place whenever the cylinder is not connected for use.
- Cylinders must be stored in ventilated areas. Closets and lockers are not acceptable storage locations. Hallways, corridors, stairwells or near elevators are also unacceptable. Additionally, cylinders of oxygen and other oxidizers must not be stored within 20-feet of fuel-gas or other combustible materials unless separated by a specific barrier, e.g., a noncombustible wall, not less than 5-feet high, having a fire-resistance rating of ½-hour. Securing devices can be purchased from any laboratory safety supply company, or the Sheet Metal Shop can develop a restraining system.
- Additional Safety Procedures – Maintain small inventories of chemicals. Large inventories are more dangerous and usually result in more wastes being generated. Store all items on secure shelves below eye level and large containers on low shelves. Never store chemicals on the floor. Storage areas should be cool, dry, ventilated and well lit. Appropriate chemical spill kits and fire extinguishers should be kept near storage areas. Containers must be sealed, capped and in good condition. Keep the outside of containers clean of chemical residue. When applicable, handling and storage procedures, outlined on MSDS, should be incorporated into your Standard Operating Procedures (SOP). Prior to working with chemicals, training on proper use and storage must be provided. If you are unsure of the correct safe handling procedures for any chemical, please contact OSEH for assistance.
Other Sources of Chemical Information
Other sources of chemical data are available from various resources, including the following:
- Sax's Dangerous Properties of Industrial Materials,
- Bretherick's Handbook of Reactive Chemical Hazards,
- The Merck Index,
- The International Technical Information Institute's Toxic and Hazardous Industrial Chemicals Safety Manual,
- ToxNet
Haz-Map was retired by the National Institute of Health (NIH) in 2022. The content of the Haz-Map is still provided by the original content provider.
Starting in December 2022, PubChem will serve as the National Library of Medicine's (NLM's) single source for chemical information. NLM has retired ChemIDplus and the Drug Information Portal, two other chemical property information sites, to better focus their development efforts on a single, integrated source of chemical information. All of the data found in ChemIDplus and the Drug Information Portal is currently available and will continue to be available in PubChem.
Chemical Spills
Hazardous substances used in laboratories require preplanning to respond safely to chemical spills. The cleanup of chemical spills should only be done by knowledgeable and experienced personnel. Spill kits with instructions, absorbents, reactants, and protective equipment should be available to clean up minor spills. A minor chemical spill is one that the laboratory staff is capable of handling safely without the assistance of safety and emergency personnel. All other chemical spills are considered to be major. Chemical spill cleanup kits, including instructions for use, are available from various laboratory safety supply vendors.
Chemical Compatibility Chart
NOTE: Identify class to which a specific compound belongs, read unsafe combinations with other classes both horizontally and vertically.
X = Unsafe Combination
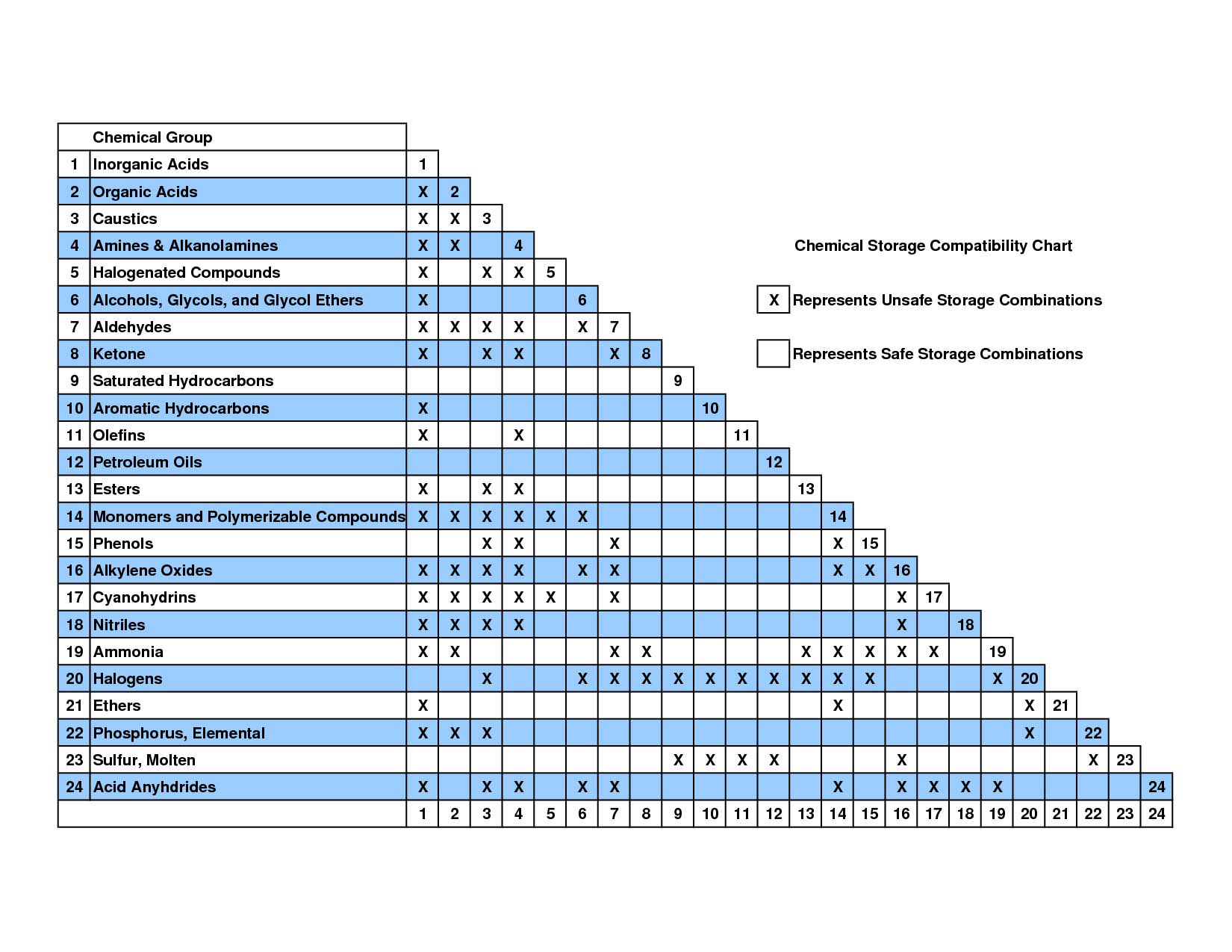
NOTE: Identify class to which a specific compound belongs, read unsafe combinations with other classes both horizontally and vertically.
X = Unsafe Combination
